Interviewer: Tell us what you do, how long you’ve done it, and what you’re good at. Matt: I’m a sustaining engineer responsible for managing production tooling and product issues. We strive to continuously improve the tooling and molding operations. I have been with C&J for 8 years and have been... read more →
Feb
18
Jan
13
This one cavity mold has four sets of interchangeable inserts, or cores, which will make multiple part versions for the customer. #plasticinjectionmolding Check out our LinkedIn for more updates like this.
Oct
27
Meet Matt Conway, Project Engineer at C&J Industries in Meadville, PA Interviewer: Hi Matt! Tell us what you do, how long you’ve done it, and what you’re good at. Matt: I’m a Project Engineer responsible for managing a customer’s project from start to finish. I’ve been with C&J Industries for... read more →
Oct
27
Congratulations to Cyrus Gage as he progresses to the Tool Shop Apprenticeship program! He completed his 1,000 hours to qualify for the program and now he'll log 7,000 more hours to complete the program. The entire program takes four years and he'll be certified as a Journeyman Tool Maker upon... read more →
Oct
27
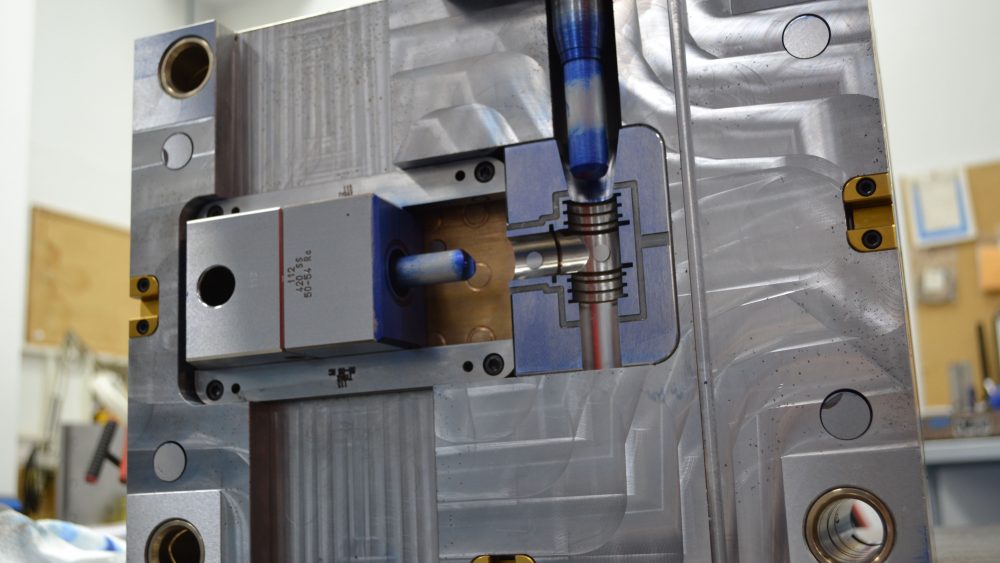
A single cavity medical mold just finished its form, fit, and function assessment. Getting ready for a “Bluing Review” before it heads to the press. A neat function of this mold is the sliding core that runs off a hydraulic cylinder.
Aug
28
Aug
27
Finishing up a form fit and function assessment on this new 16 cavity medical device mold. Notice the bluing on the plates from our wear assessment and at the end it's all cleaned up before its debut on the press tomorrow! Fun fact: this mold weights 3,845 lbs.
Aug
27
Meet Jordan Walker, Project Engineer at C&J Industries Interviewer: Tell us what you do, how long you’ve done it, and what you’re good at. Jordan: I am currently a Project Engineer that is responsible for managing a customer’s project through C&J’s internal processes and supporting the customer’s requirements to get... read more →
Aug
07
We know the value of continually investing and we believe our customers do too. As a registered FDA Medical Device Manufacturer and certified ISO 13485:2016, we are continuously improving our facility to meet and exceed those standards. Updating our floors in the Tool shop brings an added level of professional... read more →
Aug
03
Mike Yurkewicz & C&J Industries part of good article in Medical Product Outsourcing